Follow all the news from the laboratory.
AIS BIOTECH is one of the 100 start-ups to invest in, according to Challenges magazine in 2024 !
AIS Biotech celebrates its first year of existence, and has the honor of being one of the 100 start-ups to invest in, selected by Challenges
Scientific and Technical Day of the Carnot Network – Biosourced Products for the Bioeconomy!
Come and meet Cermav on May 14th, 2024 at the Scientific and Technical Day of the Carnot network – Biosourced Products for the Bioeconomy The
6th FBPOL2024 – French Brazilian Meeting on Polymers: Bridging Innovation and Collaboration in Polymer and Bio(Material) Science
After the great success and impact of previous editions, we are proud to announce the 6th Franco-Brazilian Meeting on Polymers, which will be held in
2024 PolyNat international industries forum
On February 1st and 2nd, 2024, the 6th edition of the PolyNat International Industries Forum was held in Grenoble. This annual international meeting brings together
Redox-responsive maltoheptaose-b-polystyrene nanoparticles containing zinc phthalocyanine: Formulation, photophysical properties, release kinetic and toxicity
In this article by Sami Halila and Redouane Borsali, in collaboration with the Federal University of Santa Maria (Brazil) and the Ningbo Institute of
Bernard Henrissat is elected member of the Academy of Sciences
At the end of the elections opened in 2023, the Academy of Sciences has just elected 18 new members, including our former colleague Bernard Henrissat
Electrospun Cactus Mucilage/Poly(vinyl alcohol) Nanofibers as a Novel Wall Material for Dill Seed Essential Oil (Anethum graveolens L.) Encapsulation: Release and Antibacterial Activities
This study, a collaboration work with University of Sfax and Grenoble INP, aimed to create long-lasting molecular carriers by producing electrospun nanofibers from cactus mucilage
Enantioselective membranes prepared by electrospinning of cellulose tris(3,5-dimethylphenyl carbamate) having various degrees of polymerization: effect of the DP on the morphology
Recently, we reported a proof of concept of enantioselective membrane filtration using a nonwoven membrane prepared by electrospinning of cellulose tris(3,5-dimethylphenyl carbamate) (CDMPC) synthesized from
Mini symposium Lyon : Glycomics and beyond….
Cermav will participate in the mini-symposium organized by Dr Petier Goekjan, on Friday December 8th, 2023 at the Batiment Lederer (ICBMS building, 1 rue Victor
Award ceremony for the 2023 Honoris Causa Doctorate diploma from UGA : Professor Wen-Chang Chen, Cermav collaborator !
On October 12th, 2023, the Honoris Causa 2023 Doctorate degree from Université Grenoble Alpes was awarded to our collaborator the Professor Wen-Chang Chen, Professor of
Sugars, a new weapon against infections!
Glycobiology, the study of the biological functions of carbohydrates, is a field of research in its own right, which could one day lead to new
Lénaïc Soullard thesis defense on November 30th, 2023
The thesis, entitled “Synthesis of photosensitive cellulose derivates and development of hydrogels by additive manufacturing for the design of medical devices” , was directed by
João COSAS joins Cermav as a CNRS research scientist (“Chargé de recherche CNRS”)
João COSAS is laureate of the external entrance examinations to the CNRS and, since October 1st, 2023, he has joined CERMAV as a permanent CNRS
Thirty years of scientific collaboration between the CNRS and Taiwan
On September 18th, 2023, an international conference celebrated the thirtieth anniversary of the first framework agreement between the CNRS and the Taiwanese National Science &
Glyco@Alps Scientific Day 2023
We are pleased to invite you to the glycoscience day on Friday 13 October 2023 at the MACI (339 av centrale, 38400 Saint Martin d’Hères).
Ons Dakhlaoui thesis defense on September 07th, 2023
The thesis, entitled “Improving selective DNP (SelDNP) for biomolecular applications”, was funded by the Labex ARCANE and co-directed by Sabine Hediger (MEM – CEA) and

AIS Biotech wins the i-LAB 2023 competition
The AIS BIOTECH start-up, resulting from the work of Cermav, is laureate of the i-Lab 2023 Competition of BPI France.
Antoine DESIGAUX’s thesis defense on June 27th, 2023
Antoine DESIGAUX completed his thesis at Cermav under the co-supervision of Laurent HEUX (CNRS Research Director) and Sonia MOLINA – BOISSEAU (Université Grenoble Alpes Senior lecturer). It is entitled « Development as well as mechanical and structural characterization of an anisotropic elastomer reinforced by cellulose microfibrils. ».

Creation of the new start-up resulting from the work of Cermav, AIS Biotech
AIS Biotech relies on innovative, efficient, industrializable biotechnology with low environmental impact, to develop a new class of anti-infective biomedicines. Click on the title for more information.
Moustoifa Said thesis defense on March 21th, 2023
Abstract: “In regenerative medicine, the implantation of combined of cells and hydrogels is a promising strategy to improve cell therapy. However, it is crucial to

Structural Glycoscience Summer School 2023 in Grenoble (from June 5th to 7th, 2023)
Register at https://glycoalps.univ-grenoble-alpes.fr/glyco-club/glyco-club-s-activities/structural-glycoscience-summer-school-2023-886248.kjsp
PhD position “Custom chemical glycosylation of recombinant proteins”
The aim of this PhD project is to develop a versatile chemical approach to protein glycosylation using the unique reactivity of natural but scarce amino
Design of hyaluronan-based dopant for conductive and resorbable PEDOT ink
Abstract “Conformable biocompatible conductive materials are increasingly sought for the development of bioelectronics. If additionally resorbable, they could serve for the design of transient implantable
Biomass in the Bioeconomy
Abstract: “Biomass is the physical basis of the bioeconomy, the renewable segment of the circular economy, and as a CO2-neutral part of the carbon cycle,
Extending Janus lectins architecture: Characterization and application to protocells
Abstract: “Synthetic biology is a rapidly growing field with applications in biotechnology and biomedicine. Through various approaches, remarkable achievements, such as cell and tissue engineering,
Harnessing Biobased Materials in Photosynaptic Transistors with Multibit Data Storage and Panchromatic Photoresponses Extended to Near-Infrared Band
Abstract: “Owing to ever-increasing environmental impact, nature-inspired biomimetic electronics are key to unlock the potential of developing environmentally friendly brain-like computing and biomimetic artificial-intelligence systems.
Paul Rivollier thesis defense on February 28th, 2023
Summary : “Influenza viruses are responsible for human flu epidemics and pandemics causing hundreds of thousands of deaths each year, or sometimes long-term sequelae. The infectious
Supramolecular carbohydrate-based hydrogels from oxidative hydroxylation of amphiphilic β-C-glycosylbarbiturates and α-glucosidaseinduced hydrogelation
The team “Self-assembly of Glycopolymers” of CERMAV has developed a new family of sugar derivatives through an eco-responsible process which is able to self-assemble into hierarchical glyconanostructures to ultimately gel water and the gelation can be triggered by the action of a glycosidase. Click on the title for more information.
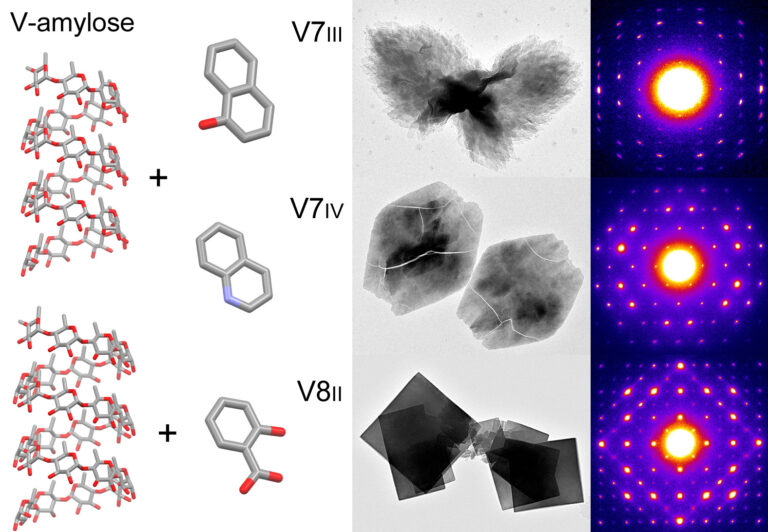
Helical inclusion complexes of amylose with aromatic compounds: Crystallographic evidence for new V-type allomorphs
The morphology and crystal structure of three new inclusion complexes of amylose with small aromatic molecules were described, thus widening the family of V-amylose allomorphs that may form when starch is processed in the presence of various ingredients and additives.
Click on the title for more information.
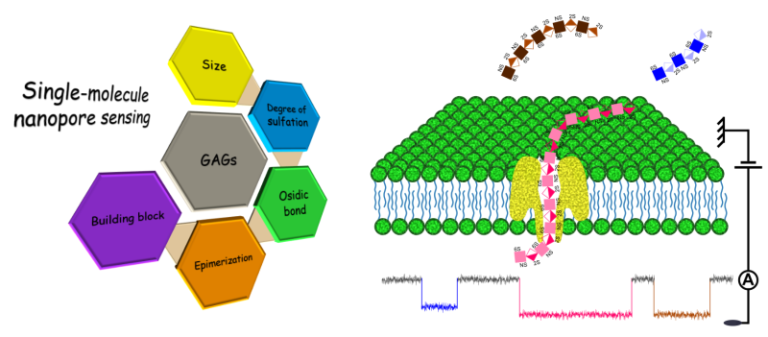
Deciphering GAGs: a new way of sequencing polysaccharides
In this publication involving Bernard Priem, Lecturer at the University of Grenoble Alpes, the authors propose an effective and robust approach based on passage through protein nanopores, to decipher the structure of complex bioactive polysaccharides, GAGs. Click on the title for more information.